In a remarkable leap forward for cardiac care, French medical device company Carmat has introduced the Aeson artificial heart. This is a total artificial heart engineered to last a lifetime, eliminating the need for donor hearts. This innovation promises to transform treatment for patients suffering from advanced biventricular heart failure, offering hope where donor organ shortages have long been a limiting factor.
How the Aeson Artificial Heart Works

The Aeson artificial heart is a sophisticated device that replaces the patient’s failing heart. It provides full circulatory support to both the left and right ventricles. Unlike previous models designed mainly as temporary “bridges” to transplantation, Aeson aims for long-term, if not lifelong, function.
This is not your average robotic heart. Carmat has designed the device using a combination of bioprosthetic and synthetic materials. Its core consists of biological tissues treated to minimize immune rejections and blood clotting, integrated with durable mechanical components to withstand years of continuous operation.
An Huge Advancement in Technology
A key technological advancement is the use of specialized vascular grafts developed in collaboration with the French company Vygon. These grafts connect the artificial heart’s outflow tracts directly to the patient’s pulmonary arteries and aorta, ensuring smooth blood flow in and out of the device. Embedded sensors continuously monitor pressure and blood flow, allowing the device to adjust pumping rates dynamically. This feedback mechanism aims to mimic the natural variability of the human heartbeat, improving physiological compatibility and patient quality of life.
Longevity: A Lifetime Solution
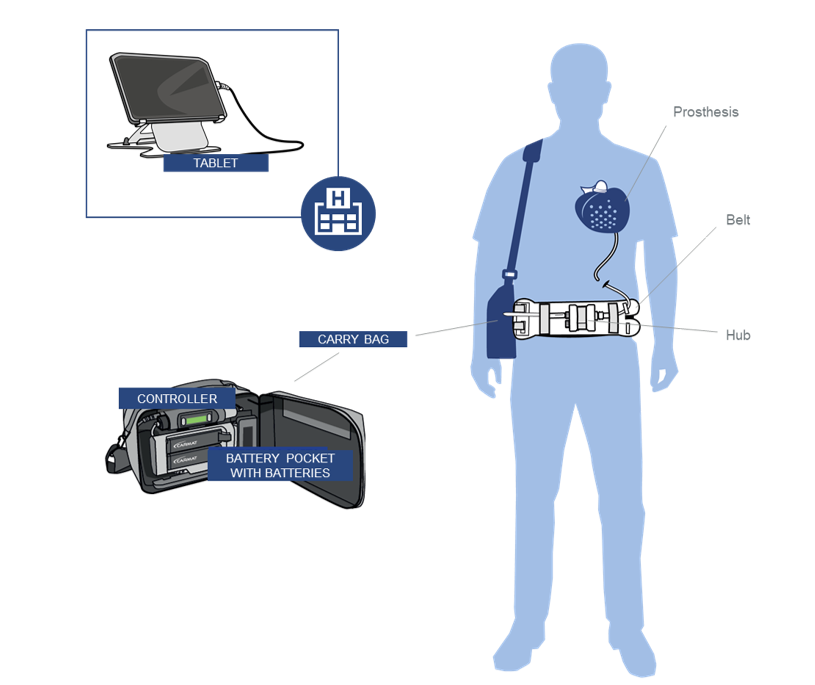
One of Aeson’s most revolutionary features is its potential durability. Over 100 implants have been performed worldwide since the first human implantation in 2013, with recent versions designed for extended wear. While traditional artificial hearts serve as temporary bridges to transplantation, Carmat’s design prioritizes a permanent solution.
Safety For Users
Current clinical studies, including early feasibility trials authorized by the US Food and Drug Administration (FDA), are assessing the device’s safety and effectiveness in patients eligible for transplantation. Initial results indicate both promising survival rates and reduced complications. This supports the device’s long-term viability.
Read More: China Unveils Smart ‘Band-Aid’ That Wirelessly Delivers Medicine to Organs on Demand
Path to Worldwide Availability
In 2021, Aeson made its US debut with the first implant at Duke University Hospital, marking a critical step toward regulatory approval across major markets. Carmat is progressing with an FDA-approved early feasibility study and plans to seek full regulatory clearance for the latest device version in the US by mid-decade. Industry analysts project a potential commercial launch in the United States as early as 2028. This regulatory momentum, coupled with increasing implant numbers and improved device iterations, indicates that Aeson could soon become a standard treatment option for patients with end-stage heart failure globally.
Implications for Heart Failure Treatment
The Aeson artificial heart represents a paradigm shift. By removing reliance on scarce donor hearts, it has the potential to dramatically reduce wait times and mortality for patients awaiting transplant. Its biocompatible design and adaptive pumping offer enhanced patient outcomes compared to existing mechanical support devices. As clinical trials continue and the device moves toward broader availability, Aeson could redefine heart failure therapy, transforming it from a race against donor supply to a sustainable, lifelong treatment.
Struggling For Funds
Unfortunately, the research and development of an artificial heart such as this requires a lot of funds. Carmat has recently filed for insolvency after failing to secure critical emergency funding. This has led to a suspension of its shares pending court decisions. Despite previously warning of cash shortages and a need to raise millions of euros to continue operations, Carmat was unable to obtain the necessary financing. This has cast uncertainty over its future, and of course, over the Aeson artificial heart, as well. This financial distress potentially impacts the further development, commercialization, and availability of the Aeson artificial heart. The company’s fate may depend on a takeover by an industrial partner or significant financial backing, with the French state’s intervention considered unlikely given the liabilities. As a result, patients and healthcare providers anticipating broader access to this groundbreaking technology may face delays or disruptions while Carmat’s future is determined in court.
The Bottom Line
Carmat’s Aeson artificial heart embodies cutting-edge cardiac science, integrating bioprosthetics, vascular engineering, and sensor-driven control to replicate natural heart function. With over a decade of clinical experience and growing support from regulatory agencies, it stands poised to revolutionize the future of heart failure care, offering hope and longevity to patients worldwide. This, of course, is only if they can find a solution to their current funding problems. Otherwise, this incredible technology will sit idle while it could be on its way to saving lives.
Read More: For the First Time Ever, Man Walks Out Of Hospital With Artificial Heart
